Two papers were recently published by researchers at the Northen Lab exploring bond breakage in phenolic compounds and the detection of glycosyl hydrolase and lignin modifying enzymes. Dr. Kai Deng, who is an author on both papers, shared some figures and highlights from these publications.
Experimental and theoretical insights into the effects of pH on catalysis of bond-cleavage by the lignin peroxidase isozyme H8 from Phanerochaete chrysosporium
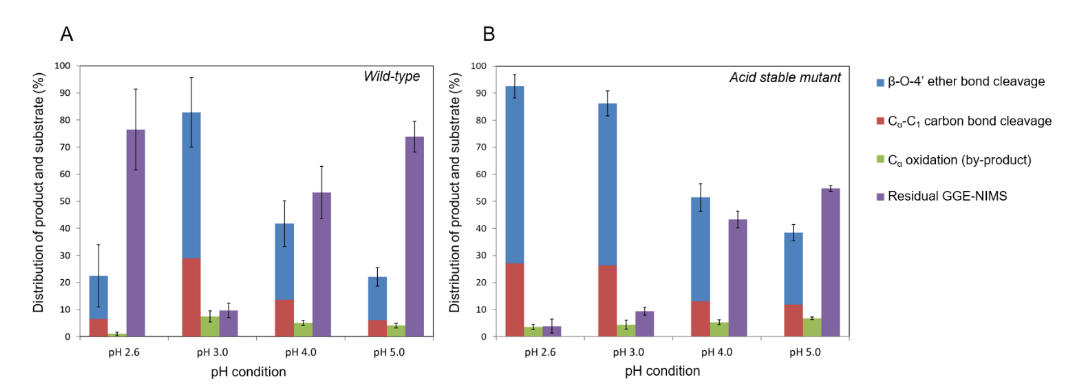
Image: Product distribution from bond by (A) wildtype lignin peroxidase and (B) acid-stabilized mutant
Using our recently developed assay based on nanostructure initiator mass spectrometry (NIMS), we presented a detailed study of the degradation of a phenolic β-O-4 dimeric model compound by lignin peroxidase isozyme H8 as a function of pH. This NIMS based assay allowed us to conduct quantitation of dimeric conversion by measuring the distribution of various products. It was found that the distribution of products is pH-dependent and that lower pH enhances bond-breaking reactions (e.g. cleavage of carbon-carbon single bond and carbon-oxygen ether bond). Ab initio molecular dynamic simulations confirmed that lower pH helps bond cleavage, especially ether bonds.
This work generates a more robust fundamental understanding of how pH assists bond cleavage and provides insights into the optimal design of reaction conditions to improve the efficiency of lignin peroxidase-catalyzed phenolic lignin degradation. Northen Lab members Kai Deng and Trent Northen contributed to this work.
Reference:
Pham, L., Deng, K., Northen, T.R. et al. Experimental and theoretical insights into the effects of pH on catalysis of bond-cleavage by the lignin peroxidase isozyme H8 from Phanerochaete chrysosporium. Biotechnol Biofuels 14, 108 (2021). https://doi.org/10.1186/s13068-021-01953-7
A multiplexed nanostructure-initiator mass spectrometry (NIMS) assay for simultaneously detecting glycosyl hydrolase and lignin modifying enzyme activities
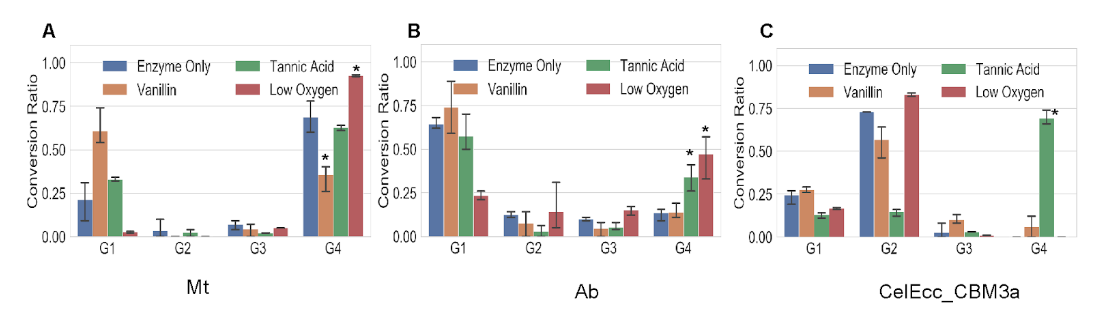
Image: Effect of mediators and inhibitory conditions on cellotetraose degradation
In this work, we demonstrated a multiplexed NIMS-based assay which enables characterization analysis of commercial laccase enzyme mixtures against lignin and glycan model compounds. Using a time-series analysis, we determined that crude laccase from Ab has higher GH activity and that laccase from Mt has the higher activity against our lignin model compound. Inhibitor studies showed a significant reduction in Mt GH activity under low oxygen conditions and increased activities in the presence of vanillin (common GH inhibitor).
These assays enable simultaneous analyses of both glycosyl hydrolase and lignin modifying enzyme activities, which we anticipate will be helpful in developing enzyme cocktails for lignocellulose deconstruction. Importantly, we find that our assay is suitable for examining the inhibition of GH activities by aromatics. This is an important consideration in the development of economically viable biomass to biofuels approaches. Northen Lab members Nicole Ing, Kai Deng, Ben Bowen, and Trent Northen contributed to this work.
Reference:
Ing, N., Deng, K., Chen, Y., Aulitto, M., Gin, J.W., Phan, T.L.M., Petzold, C.J., Singer, S.W., Bowen, B., Sale, K.L., Simmons, B.A., Singh, A.K., Adams, P.D., and Northen, T.R. A multiplexed nanostructure-initiator mass spectrometry (NIMS) assay for simultaneously detecting glycosyl hydrolase and lignin modifying enzyme activities. Sci Rep 11, 11803 (2021). https://doi.org/10.1038/s41598-021-91181-8